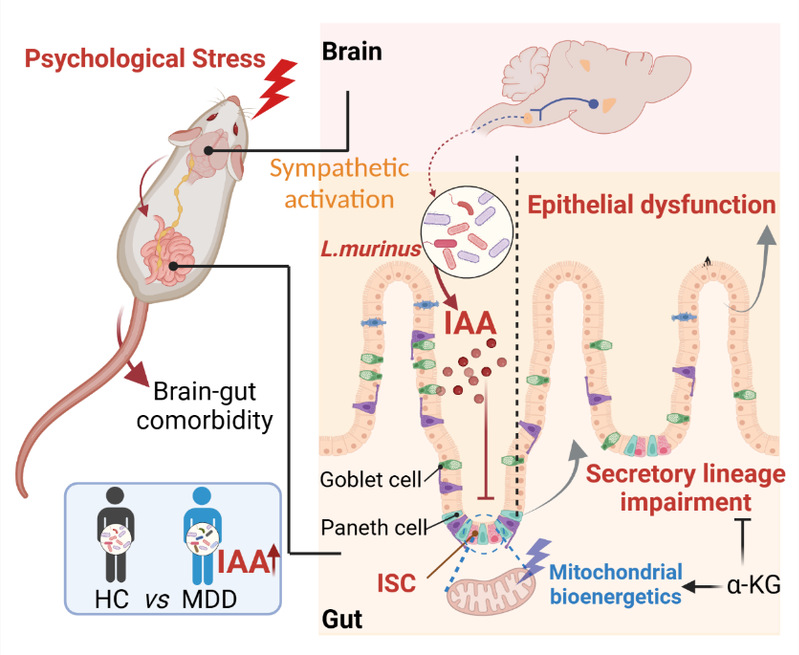
Cell Metabolism published online the latest research result of Prof. Hao Haiping's team entitled Psychological Stress-induced Microbial Metabolite Indole-3-acetate Disrupts Intestinal Cell Lineage Commitment. The study reveals a new mechanism of brain-gut signaling and metabolic regulation of chronic mental stress-induced susceptibility to intestinal epithelial cell injury, and discovers gut microbiota markers with clinical monitoring value. Wei Wei (Doctoral student), Liu Yali (Master’s degree graduate), and Hou Yuanlong (Postdoctoral fellow) from our School of Pharmacy are co-first authors of this paper. Prof. Hao Haiping, Academician Wang Guangji, Prof. Zheng Xiao, and Prof. Yuan Yonggui of Southeast University serve as co-corresponding authors. and our university is the primary affiliation. The paper was designated as the cover article in the March issue of Cell Metabolism and the research progress was featured in a NEWS update on the Nature homepage. Epidemiological studies indicate that long-term exposure to psychosomatic stress contributes to various diseases, including a high prevalence of intestinal disorders such as inflammatory bowel disease and irritable bowel syndrome. Despite this, the brain-gut bidirectional signal communication under mental stress remains poorly understood, particularly regarding how mental stress disrupts intestinal homeostasis.
Based on previously established chronic restraint stress (CRS) mouse model and plantar electric shock model Targeting the above issues, Prof. Hao’s team found that chronic stress exposure triggers irreversible small intestinal epithelial cell dysfunction. Through organoid culture, intestinal stem cell lineage-tracing and multi-omics approaches, the team discovered that these phenotypes are associated with the disruption of mitochondrial bioenergetics and functional homeostasis in small intestinal stem cells (ISCs) under mental stress. The team further found that L. murinus and its metabolite, indole-3-acetic acid (IAA), play a key role in the stress-induced ISC differentiation abnormalities through chemical intervention, sterile mice, metabolomics and engineered bacteria construction. Subsequent mechanistic studies showed that IAA functions as a metabolic signal that inhibits ISC mitochondrial bioenergetics, leading to impaired lineage differentiation and exacerbating damage to intestinal epithelial cell in a cell-intrinsic manner. However, supplementation with the tricarboxylic acid cycle intermediate, α-ketoglutaric acid, effectively countered IAA-induced damage both in vitro and in vivo. Finally, the team demonstrated that psychiatric stress consistently enhances the IAA-producing capacity of gut flora, which is linked to intestinal dysfunction in clinical patients with different psychiatric disorders. This study identifies the signaling molecules and mechanisms underlying microbial dysregulation that drive intestinal stem cell differentiation abnormalities under mental stress using multidisciplinary approaches. It offers new insights and potential targets for future research on brain-gut axis signaling, target identification, and precision drug intervention.
This research work was funded by the National Key R&D Program, the Science Fund for Creative Research Groups of the National Natural Science Foundation of China, the National High-level Young Talent Program, and the Pharmacy Scholars Program of our university. This study had also received substantial supports from the State Key Laboratory of Natural Medicines and the Jiangsu Key Laboratory of Pharmacokinetics.
Original article: https://www.cell.com/cell-metabolism/fulltext/S1550-4131(23)00477-1
Link to Nature's official website story: https://www.nature.com/articles/d41586-024-00188-4
Figure
(Source:School of Pharmacy,Written by: Liu Hua, Translated by: Gan Jue)


