1. Research Projects
(1)National Center of Technology Innovation for Biopharmaceuticals, “Multi-barrier breaking based oral nanovaccine in gene delivery”, 2022-2025, 500,000 RMB
(2)Double First-Class Program of China Pharmaceutical University, “Intestinal lymphatic transport-based oral lipid nanovaccine in gene delivery”, 2022-2025, 200,000 RMB
(3)China Pharmaceutical University-Shandong Xinhua Pharmaceutical Joint Development of Major Innovative Drugs and High-end Preparations, 2021-2030, 30,000,000 RMB
(4)Development of innovative pharmaceutical formulations, 2018-2038, 10,000,000 RMB
(5)Development of compound hypoglycemic drug sustained-release tablets, 2020-2030, 7,000,000 RMB
(6)National Natural Science Foundation of China, “Targeted prodrug and liposome hybridization system-mediated chemotherapy-immunotherapy and Mechanistic study”, 2019-2022, 570,000 RMB
(7)National Natural Science Foundation of China, “Study on the role and mechanism of miRNA cytoplasmic direct delivery system based on poorly soluble drug nanocrystals”, 2017-2020, 600,000 RMB
(8)National Natural Science Foundation of China, “Efficacy and mechanism of thermo-sensitive liposomes targeting MMPs and CD44 receptor in cancer stem cells”, 2015-2018, 700,000 RMB
2. Academic Awards
(1)Excellent scientific and technological innovation team in universities, 2021, Rank first
(2)First prize of Jiangsu Province Science and Technology Progress Award, “Key technologies and applications of sustained-release intelligent drug delivery systems”, 2019, Rank first
(3)The New Century Excellent Talent Support Program of the Ministry of Education
(4)The innovation team leader of Qinglan Program for the Department of Education of Jiangsu
(5)The second level mid-aged leader of Science and Technology in Jiangsu 333 Program
(6)The outstanding mid-aged experts in Nanjing
3. Representative Research Achievements
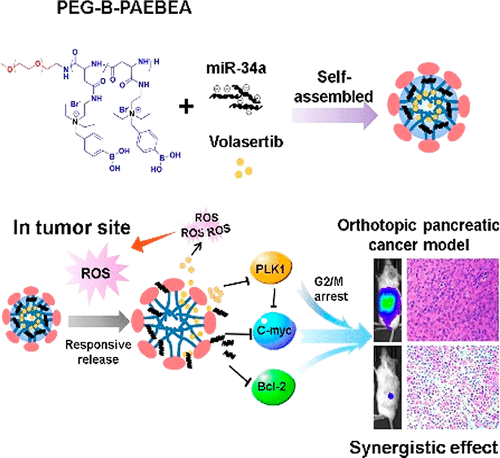
(1)Ineffective drug delivery and poor prognosis are two major challenges in the treatment of pancreatic ductal adenocarcinoma (PDAC). While there is significant downregulation of tumor suppressor microRNA-34a (miR-34a), which targets many oncogenes related to proliferation, apoptosis, and invasion, high expression level of Polo-like kinase 1 (PLK1) is closely associated with short survival rates of pancreatic cancer patients. Therefore, the objective is to codeliver miR-34a mimic and small molecule PLK1 inhibitor volasertib using poly(ethylene glycol)-poly[aspartamidoethyl(p-boronobenzyl)diethylammonium bromide]. This polymer could self-assemble into micelles of ∼100 nm with 10% drug loading of volasertib and form a complex with miR-34a at the N/P ratio of 18 and higher. Combination treatment of volasertib and miR-34a displayed the synergistic effect and superior antiproliferative activity along with an enhanced G2/M phase arrest and suppression of colony formation, leading to cell death due to potential c-myc targeting therapeutics. Orthotopic pancreatic tumor bearing NSG mice were scanned for fluorescence by IVIS after systemic administration of micelles encapsulating volasertib and miR-34a. There was significant reduction in tumor volume, and histological examination of major organs suggested negligible systemic toxicity. In conclusion, PEG-B-PAEBEA micelles carrying volasertib and miR-34a mimic have the potential to treat pancreatic cancer.
(2)Strong infectivity enables coronavirus disease 2019 (COVID-19) to rage throughout the world. Remdesivir is one of the most promising anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drugs. We developed lyophilized remdesivir liposomes (Rdv-lips) which can be reconstituted as liposomal aerosol for pulmonary delivery to improve the in vivo behavior of existing remdesivir cyclodextrin conclusion compound (Rdv-cyc) injections. The in vitro liposomal aerosol characterization demonstrated that Rdv-lips possessed a mass median aerodynamic diameter of 4.118 µm and fine particle fraction (<5 µm) higher than 50%, indicating good pulmonary delivery properties. Compared to the Rdv-cyc intravenous injection group, the Rdv-lips inhalation group displayed a nearly 100-fold increase in the remdesivir-active metabolite nucleotide triphosphate (NTP) concentration and better NTP accumulation in the lung than the Rdv-cyc inhalation group. A faster transition from remdesivir to NTP of Rdv-lips (inhalation) could also be observed due to better cell uptake. Compared to other preparations, the superiority of Rdv-lips was further evidenced by the results of an in vivo safety study, with little possibility of inducing inflammation. In conclusion, Rdv-lips for pulmonary delivery will be a potent formulation to improve the in vivo behavior of remdesivir and exert better therapeutic effects in COVID-19 treatment.

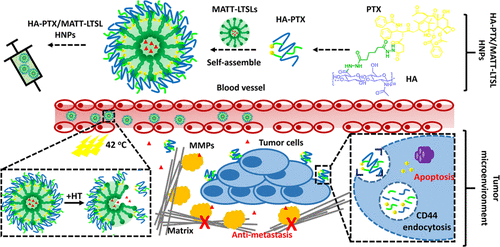
(3)The tumor microenvironment plays a critical role in tumor initiation, progression, invasion, and metastasis. Therefore, a therapy that combines chemotherapeutic drugs with a TME modulator could be a promising route for cancer treatment. This paper reports a nanoplatform self-assembled from a hyaluronic acid-paclitaxel (HA-PTX) prodrug and marimastat-loaded thermosensitive liposomes (LTSLs) (MATT-LTSLs) for the dual targeting of the TME and cancer cells. Interestingly, the prodrug HA-PTX can self-assemble on both positively and negatively charged liposomes, forming hybrid nanoparticles (HNPs). Triggered by mild hyperthermia, HA-PTX/MATT-LTSLs HNPs rapidly release their payloads into the extracellular environment, and the released HA-PTX quickly enters 4T1 cells through a CD44-HA affinity. The HNPs possess promoted tumor accumulation, exhibit deep tumor penetration, and significantly inhibit the tumor growth, metastasis, and angiogenesis. Importantly, by targeting the TME and maintaining its integrity via inhibiting the expression and activity of matrix metalloproteinases, blocking the fibroblast activation by downregulating the TGF-β1 expression and suppressing the degradation of extracellular matrix, the HNPs allow for significant metastasis inhibition. Overall, these findings indicate that a prodrug of an HA–hydrophobic-active compound and liposomes can be self-assembled into a smart nanoplatform for the dual targeting of the TME and tumor cells and efficient combined treatment.
Nanomedicine for gene delivery, Nanovaccine, Tumor targeting, Metabolic disease, Autoimmune disease, Chemo-immunotherapy, Formulation development and industrialization, Interpretation of regulatory policies, Compliance with drug registration
1. Research Projects
(1)National Center of Technology Innovation for Biopharmaceuticals, “Multi-barrier breaking based oral nanovaccine in gene delivery”, 2022-2025, 500,000 RMB
(2)Double First-Class Program of China Pharmaceutical University, “Intestinal lymphatic transport-based oral lipid nanovaccine in gene delivery”, 2022-2025, 200,000 RMB
(3)China Pharmaceutical University-Shandong Xinhua Pharmaceutical Joint Development of Major Innovative Drugs and High-end Preparations, 2021-2030, 30,000,000 RMB
(4)Development of innovative pharmaceutical formulations, 2018-2038, 10,000,000 RMB
(5)Development of compound hypoglycemic drug sustained-release tablets, 2020-2030, 7,000,000 RMB
(6)National Natural Science Foundation of China, “Targeted prodrug and liposome hybridization system-mediated chemotherapy-immunotherapy and Mechanistic study”, 2019-2022, 570,000 RMB
(7)National Natural Science Foundation of China, “Study on the role and mechanism of miRNA cytoplasmic direct delivery system based on poorly soluble drug nanocrystals”, 2017-2020, 600,000 RMB
(8)National Natural Science Foundation of China, “Efficacy and mechanism of thermo-sensitive liposomes targeting MMPs and CD44 receptor in cancer stem cells”, 2015-2018, 700,000 RMB
2. Academic Awards
(1)Excellent scientific and technological innovation team in universities, 2021, Rank first
(2)First prize of Jiangsu Province Science and Technology Progress Award, “Key technologies and applications of sustained-release intelligent drug delivery systems”, 2019, Rank first
(3)The New Century Excellent Talent Support Program of the Ministry of Education
(4)The innovation team leader of Qinglan Program for the Department of Education of Jiangsu
(5)The second level mid-aged leader of Science and Technology in Jiangsu 333 Program
(6)The outstanding mid-aged experts in Nanjing
3. Representative Research Achievements
(1)Ineffective drug delivery and poor prognosis are two major challenges in the treatment of pancreatic ductal adenocarcinoma (PDAC). While there is significant downregulation of tumor suppressor microRNA-34a (miR-34a), which targets many oncogenes related to proliferation, apoptosis, and invasion, high expression level of Polo-like kinase 1 (PLK1) is closely associated with short survival rates of pancreatic cancer patients. Therefore, the objective is to codeliver miR-34a mimic and small molecule PLK1 inhibitor volasertib using poly(ethylene glycol)-poly[aspartamidoethyl(p-boronobenzyl)diethylammonium bromide]. This polymer could self-assemble into micelles of ∼100 nm with 10% drug loading of volasertib and form a complex with miR-34a at the N/P ratio of 18 and higher. Combination treatment of volasertib and miR-34a displayed the synergistic effect and superior antiproliferative activity along with an enhanced G2/M phase arrest and suppression of colony formation, leading to cell death due to potential c-myc targeting therapeutics. Orthotopic pancreatic tumor bearing NSG mice were scanned for fluorescence by IVIS after systemic administration of micelles encapsulating volasertib and miR-34a. There was significant reduction in tumor volume, and histological examination of major organs suggested negligible systemic toxicity. In conclusion, PEG-B-PAEBEA micelles carrying volasertib and miR-34a mimic have the potential to treat pancreatic cancer.

(2)Strong infectivity enables coronavirus disease 2019 (COVID-19) to rage throughout the world. Remdesivir is one of the most promising anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drugs. We developed lyophilized remdesivir liposomes (Rdv-lips) which can be reconstituted as liposomal aerosol for pulmonary delivery to improve the in vivo behavior of existing remdesivir cyclodextrin conclusion compound (Rdv-cyc) injections. The in vitro liposomal aerosol characterization demonstrated that Rdv-lips possessed a mass median aerodynamic diameter of 4.118 µm and fine particle fraction (<5 µm) higher than 50%, indicating good pulmonary delivery properties. Compared to the Rdv-cyc intravenous injection group, the Rdv-lips inhalation group displayed a nearly 100-fold increase in the remdesivir-active metabolite nucleotide triphosphate (NTP) concentration and better NTP accumulation in the lung than the Rdv-cyc inhalation group. A faster transition from remdesivir to NTP of Rdv-lips (inhalation) could also be observed due to better cell uptake. Compared to other preparations, the superiority of Rdv-lips was further evidenced by the results of an in vivo safety study, with little possibility of inducing inflammation. In conclusion, Rdv-lips for pulmonary delivery will be a potent formulation to improve the in vivo behavior of remdesivir and exert better therapeutic effects in COVID-19 treatment.

(3)The tumor microenvironment plays a critical role in tumor initiation, progression, invasion, and metastasis. Therefore, a therapy that combines chemotherapeutic drugs with a TME modulator could be a promising route for cancer treatment. This paper reports a nanoplatform self-assembled from a hyaluronic acid-paclitaxel (HA-PTX) prodrug and marimastat-loaded thermosensitive liposomes (LTSLs) (MATT-LTSLs) for the dual targeting of the TME and cancer cells. Interestingly, the prodrug HA-PTX can self-assemble on both positively and negatively charged liposomes, forming hybrid nanoparticles (HNPs). Triggered by mild hyperthermia, HA-PTX/MATT-LTSLs HNPs rapidly release their payloads into the extracellular environment, and the released HA-PTX quickly enters 4T1 cells through a CD44-HA affinity. The HNPs possess promoted tumor accumulation, exhibit deep tumor penetration, and significantly inhibit the tumor growth, metastasis, and angiogenesis. Importantly, by targeting the TME and maintaining its integrity via inhibiting the expression and activity of matrix metalloproteinases, blocking the fibroblast activation by downregulating the TGF-β1 expression and suppressing the degradation of extracellular matrix, the HNPs allow for significant metastasis inhibition. Overall, these findings indicate that a prodrug of an HA–hydrophobic-active compound and liposomes can be self-assembled into a smart nanoplatform for the dual targeting of the TME and tumor cells and efficient combined treatment.

1. Xiaofei Xina,#, Jingjing Lia,#, Wantao Wua,#, Lianjie Rena,d, Pengbo Zhaoa, Ying Zhua, Chao Qina,*, Lifang Yina,b,c,* “ROS-scavenging Nanomedicine for “Multiple Crosstalk” Modulation in Non-alcoholic Fatty Liver Disease”. Biomaterials science. 2023, accepted. (IF 7.590)
2. Zhou, Yong, Lei Yang, Yifu Lyu, Di Wu, Ying Zhu, Jingjing Li, Dabo Jiang, Xiaofei Xin*, and Lifang Yin*. Topical Delivery of ROS-Responsive Methotrexate Prodrug Nanoassemblies by a Dissolvable Microneedle Patch for Psoriasis Therapy. International Journal of Nanomedicine (2023): 899-915. (IF 7.033)
3. Xiaofei Xin, Yong Zhou, Jingjing Li, Kai Zhang, Chao Qin*, and Lifang Yin*. CXCL10-coronated thermosensitive “stealth” liposomes for sequential chemoimmunotherapy in melanoma. Nanomedicine: Nanotechnology, Biology and Medicine 48 (2023): 102634. (IF: 6.458)
4. Lianjie Ren, Jingjing Li, Lisha Liu, Wantao Wu, Di Zhao, Kai Zhang, Xiaofei Xin, Lei Yang, Lifang Yin*. “Resolving hepatic fibrosis via suppressing oxidative stress and an inflammatory response using a novel hyaluronic acid modified nanocomplex.” Biomater Sci 9, no 24 (2021): 8259-8269. (IF: 7.590)
5. Li, Jingjing, Kai Zhang, Di Wu, Lianjie Ren, Xinyu Chu, Chao Qin, Xiaopeng Han, Taijun Hang, Yungen Xu, Lei Yang, Lifang Yin*. Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19. Asian journal of pharmaceutical sciences 16, no. 6 (2021): 772-783. (IF: 9.289)
6. Chao Teng, Beiyuan Zhang, Zhongyue Yuan, Zheng Kuang, Zhuodong Chai, Lianjie Ren, Chao Qin, Lei Yang, Xiaopeng Han, and Lifang Yin*. Fibroblast activation protein-α-adaptive micelles deliver anti-cancer drugs and reprogram stroma fibrosis. Nanoscale 12, no. 46 (2020): 23756-23767. (IF: 7.79)
7. Zhuodong Chai, Chao Teng, Lei Yang, Lianjie Ren, Zhongyue Yuan, Siyuan Xu, Manman Cheng, Yanmei Wang, Zhen Yan, Chao Qin, Xiaopeng Han, Lifang Yin*. Doxorubicin delivered by redox-responsive Hyaluronic Acid–Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydrate polymers 245 (2020): 116527. (IF: 10.723)
8. Xiaofei Xin, Xiaoqing Du, Qingqing Xiao, Helena S.Azevedo, Wei He, Lifang Yin*. Drug Nanorod-Mediated Intracellular Delivery of microRNA-101 for Self-sensitization via Autophagy Inhibition. Nano-Micro Letters, 2019,11(04):453-468. (IF: 23.655)
9. Xiaofei Xin, Feng Lin, Qiyue Wang, Lifang Yin*, Ram I. Mahato*. ROS-Responsive Polymeric Micelles for Triggered Simultaneous Delivery of PLK1 Inhibitor/miR-34a and Effective Synergistic Therapy in Pancreatic Cancer. ACS applied materials & interfaces,2019,11(16):14647-14659. (IF: 10.383)
10. Yaqi Lv, Chaoran Xu, Xiangmei Zhao, Chenshi Lin, Xin Yang, Xiaofei Xin, Li Zhang, Chao Qin, Xiaopeng Han, Lei Yang, Wei He, and Lifang Yin*. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 12, no. 2 (2018): 1519-1536. (IF: 15.881)
11. Xiaofei Xin, Xue Pei, Xin Yang, Yaqi Lv, Li Zhang, Wei He, and Lifang Yin*. Rod-Shaped Active Drug Particles Enable Efficient and Safe Gene Delivery. Advanced Science 4, no. 11 (2017): 1700324. (IF: 15.444)
1. Lab director: | ||
| ||
Lifang Yin, PhD, professor | ||
2. Associate Directors: | ||
|
| |
Chao Qin, PhD, Associate professor | Xiaopeng Han, PhD, Associate professor | |
|
| |
Lei Yang, PhD, Associate professor | Xiaofei Xin, PhD, Research associate | |
|
| |
Lisha Liu, PhD, Research associat | Zhen Yan, Master, Research assistant | |
|
| |
Yuhong Chen, Master, Research assistant | Qiong Zhu, Master, Research assistant | |
3. Staff: | ||
|
|
|
Jingjing Zhao, Lab manager | Fangmei Liang, Research assistant | Yuting Chen, Research assistant |
4. Post-doctoral fellow: | ||
| ||
Yanming Xia, Post-doctoral fellow | ||
5. PhD students: | ||
|
|
|
Yong Zhou (2019-) | Jingjing Li (2019- | Yifu Lyu (2021-) |
|
|
|
Beiyuan Zhang (2021-) | Taiyu Liu (2022-) | Di Wu (2023-) |
And Siyuan Xu, Gefei Meng, Chunli Zhu, Feng Su, Chunxia Zhu, Jintuo Chen, Shenye Zhang, Lijun Gao. | ||
6. Graduate students: | ||
Chuanhong Shen, Huilin Jin, Si Chen, Haoxuan Dou, Lanlan Liu, Wantao Wu, Jiarui Yan, Yuhe Zhao, Peng Lin, Huangqin Hu, Yuan Jiang, Yourong Yin, Meixi Jin, Jiameng Xing, Yifu Sun, Xi Lin, Zhanlei Wang, Xianggui Wu, Lixi Hu, Mengqi Jiang, Dabo Jiang, Yiyang Zhou, Wenyan Zhang, Yuxuan Gao, Li Zeng, Yunhan Xia, Shuxian Xu, Yuanyuan Li, Qiushi Li, Yuqing Liu, Yixuan Wang, Mengyao Cui, Jianyuan Jiang, Ruining Liu. | ||






















