Recently, the internationally renowned journal Angewandte Chemie International Edition (IF: 16.6) published online the latest scientific research findings of Prof. Yao Hequan/Gao Shang’s team from our school titled Copper Catalyzed Enantioselective and Regiodivergent Allylation of Ketones with Allenylsilanes. Postgraduate student Xu Menghua and Lu Qingbin at CPU are the co-first authors of this paper. Prof. Yao Hequan and Associate Researcher Gao Shang from our school are the co-corresponding authors. CPU is the unique correspondence affiliation.
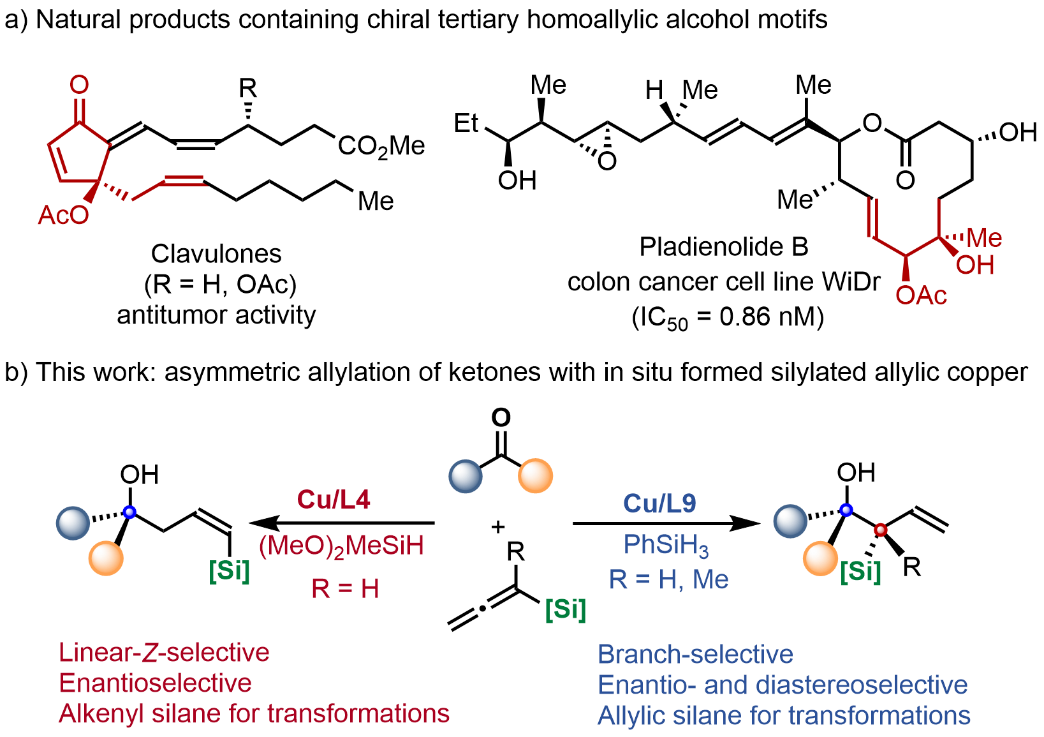
Polyketides, an important class of natural products generated from bacteria, fungi, and plants, exhibit extensive medicinal value and important physiological functions. These compounds often contain many chiral tertiary homoallylic alcohol motifs, multiple chiral centers, and stereo-defined olefinic fragments. Due to the existence of complex and diverse stereoisomers of these natural products, drug research and development work is extremely challenging. On the other hand, the stereochemistry configuration of chiral drugs has an extremely important impact on their biological activity. Different stereoisomers present sharp differences in biological activity, and further, some have totally different pharmacological activities. Therefore, achieving efficient and precise synthesis of tertiary homoallylic alcohols will greatly assist the drug discovery and development of bioactive polyketides.
In recent years, targeting the construction of molecular fragments and building blocks of bioactive drugs, Prof. Yao and Gao’s team has designed and synthesized a variety of chiral molecular skeletons through transition-metal-catalyzed hydrofunctionalization and other strategies. They have achieved several important research results (J. Am. Chem. Soc. 2022, 144, 11364-11376; Angew. Chem. Int. Ed. 2023, 62, e202305518). Based on this, the team has recently designed and developed a copper-catalyzed enantioselective and regiodivergent allylation of ketones with allenylsilanes. In this study, high regioselective and enantioselective syntheses of branched tertiary homoallylic alcohols as well as linear-Z-type products have been achieved by using different chiral ligands, respectively. In addition, through the palladium catalyzed double bond isomerization strategy, the linear-Z-products can be smoothly converted into the linear-E-products. Alkenylsilane or allylsilane fragments in the products can directly participate in various transformations, enriching the structural diversity of the products quickly and efficiently.
These researches have been supported by the General Program of the National Natural Science Foundation of China (NSFC22071267, NSFC22371299), the Independent Project of the State Key Laboratory of API of Natural Products (SKLNMZZ202211), and the Youth Fund of the Natural Science Foundation of Jiangsu Province (BK20210414).
Original link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202311540